Training course MEDICAL DEVICES : FROM DESIGN TO MARKET
Informations :Xavier Garric
MDDM COURSE (in French DMCC)
MEDICAL DEVICES : FROM DESIGN TO MARKET
A transversal training to an extensive knowledge of the medical device sector
Xavier Garric, responsable du parcours DMCC
SKILLS AND KNOWLEDGE BY THE END OF THE COURSE
- General knowledge of the applications of medical devices by medical discipline,
- Extensive knowledge of regulatory and economic constraints of the MD market,
- Ability to design a medical device based on scientific and technical skills on biomaterials,
- Mastering the specificities of the medical device throughout its lifecycle in the company (R&D, production, Regulatory, Quality, Marketing…).
SPEAKERS
- Biomaterials specialists (researchers and professors),
- Hospital practitioners, Health institutions representatives (ANSM, HAS, CNEDiMTS, CEPS…),
- Industrial experts from different sectors of the medical device..
BUSINESS OPPORTUNITIES
Project Manager in different domains of the medical device industry :
R&D • Production • Regulatory Affairs Quality • Pre-clinical and clinical evaluations of medical devices
PROFESSIONAL EXPERIENCES
INTERNSHIP : 5 MONTHS IN MASTER 1 AND 6 MONTHS IN MASTER 2 OR APPRENTICESHIP TRAINING
Manon and Nicolas, MDDM master students present their formation(FR)
Damien Stefanelli, Quality Supervisor (Maquet, Getine Group), presents the MDDM with the industry point of view (FR)
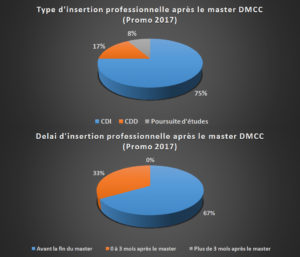
integration into the workplace for MDDM students in 2017 (FR)
Les étudiants du Master 2 parcours DMCC expliquent leurs choix de formation et leur vision pour l’avenir.
OBJECTIVES AND CONTENTS OF THE MASTER COURSE :
MASTER 1
SEMESTER 1
COMMON CORE (S1)
TU 101 : REGULATORY AND FINANCIAL ASPECTS – QUALITY AND SAFETY
Goals:
Pharmaceutical law:
- To know the various texts applying to the field of medicines, their origin, their legal value, their articulation
- To have some knowledge of the various health products
- To know the basics of innovation protection control, manufacturing, marketing and monitoring of medicines
Quality :
- To know the quality demands and management tools
Security :
- To know the regulations of ICPE classified installations
- To know the classification and labelling of chemical products’ hazards
Marketing :
- To integrate the marketing’s global approach
- To know the diagnostic tools and the strategic directions
Financial analysis
- To be able to analyse the profitability, the solvency and the liquidity of an economic activity (industrial company)
Content:
- Pharmaceutical law (18hrs-Lecture)
- Financial analysis (12hrs-Lecture)
- Marketing (6hrs-Lecture)
- Quality (10hrs-Lecture)
- Safety (6hrs-Lecture)
Teachers:
- V. RAGE-ANDRIEU
- C. LE GAL
- R. BARTHES
- N. DELELIGNE
- A. ESCANDE
- V. BOISSERIE LAPORTE
- O. VEYRET
- A. GARD
TU 102: DATABASES – EPIDEMIOLOGY AND ENVIRONNEMENTAL HEALTH
Goals:
To comprehend epidemiological studies:
- To identify epidemiology as public health tools
- To know the epidemiological surveillance tools
- To know the different types of epidemiological surveys
- To be able to interpret descriptive epidemiology indicators
- To be able to calculate and interpret the results of risk calculation (RR and OR) from analytical epidemiology
- To be able to describe and criticise the strategies and protocols of studies implemented in epidemiology
- To be able to assess the validity of screening tests
- To know the behaviour and the impact of organic pollutants present in the environnement and to understand the related legislation
Databases:
- To discover the basics of databases management
- To understand how the information is structured thanks to the relational model
- To recognise the different database usage patterns (administrator/client)
- To be able to build a database including a few tables
- To be able to define the main confines on a database
- To be abe to use the main commands of the SQL language
- To be autonomous on the data management system ACCESS (Microsoft)
Content:
- Database (4hrs-Lecture+ 15hrs-Tutorial)
- Epidemiology (12hrs-Lecture)
- Environmental health (18hrs-Lecture)
Teachers:
- M. VIVIEN
- P. RAVEL
- H. FENET
- A. ESCANDE
- F. COURANT
- C. CASELLAS
- E. GOMEZ
TU 103: HEALTH PRODUCTS DEVELOPMENT STRATEGY– PROJECT MANAGEMENT AND CLINICAL DEVELOPMENT
Goals:
- General knowledge of the sectors of medicines, cosmetology and medical devices
- Knowledge of the regulatory and socio-economic specificities of the medicines, cosmetology and medical devices
- Initiation to the project management of health products development
- Initiation to the proceedings of clinical trials in accordance with the Good Clinical Practices (GCP)
Content:
- Development strategy: Medicines (12hrs-Lecture), Cosmetology (6hrs-Lecture) and Medical Devices (6hrs-Lectures)
- Regulations in Europe, Japan and the United-States (6hrs-Lecture)
- Clinical development (9hrs-Lecture)
- Project management (6hrs-Lecture + 3hrs-Tutorial)
Teachers:
Montpellier University
- B. BATAILLE
- S. BEGU
- J. COUDANE
- X. GARRIC
- A. EL GHAZOUI
- P. CREMADES
- W. AGOUMI
TU 104: BIOTECHNOLOGIES APPLIED TO HEALTH
Goals:
- To know the various health products produced by biotechnologies
- To acquire the notions of therapeutic targets and biomarkers
- To acquire the notions of medium and high throughput screening
- To know the technologies of recombinant DNA (GMO notion) and protein engineering
- To know the production and quality control processes
- To have a concrete overview of the conditions of production of a recombinant protein and of the development of a cellular model that is compatible with medium throughput screening (practical works carried out)
Content:
- Identification of the molecular or cellular targets and of the biomarkers (8hrs-Lecture)
- Production/Quality control (3hrs-Lecture)
- Industrial applications/examples (11hrs-Lecture)
- Virtual cloning (6hrs-Tutorial)
- Production, purification, characterisation of a recombinant protein (12hrs-Practical work)
- Preparation of a cellular model for medium throughput screening– visit of a high throughput pharmacological screening platform (10hrs-Practical work)
Teachers:
Montpellier University
- S. CHAUMONT
- A. CHOQUET
- F. COURANT
- S. DELBECQ
- A-D. LAJOIX
- X. GARRIC
- S. MARY
- F. MACARI-FINE
- M.A. POUL
External professionals
- ARPEGE Platform, IGF Montpellier
TU 105: FOREIGN LANGUAGES AND COMMUNICATION
Goals:
- To develop communication skills in foreign languages
- Specialized vocabulary
- Knowledge of the companies and health matters in the countries of the target language
- Cultural approach
Content:
- Foreign languages (40hrs-Tutorial)
- Communication (9hrs-Tutorial)
Teachers:
Montpellier University
- I. MAITRE-DEVALLON
- Y. ZAMBRANO
- G. NICKSON
- P. WEINMANN
External professionals
- Communication consultants
SPECIFIC TU (S1): MEDICAL DEVICES : FROM DESIGN TO MARKET
TU 109: BIOMATERIALS AND MEDICAL DEVICES
Objectives:
- Introduction to the medical device sector
- General and transversal knowledge of the different classes of biomaterials used for the design of medical devices
Content : 40h (Lecture) and 10h (Practical work)
- Introduction to medical devices
- Biomaterials : definition, biocompatibility and biofunctionality
- Prerequisite about chemical and mechanical properties
- Prerequisite about aging / degradation
- The different classes of biomaterials
- Practical work and mini-projects
Intervenants:
- H. VAN DEN BERGHE
- J. COUDANE
- B. NOTTELET
- X. GARRIC
SEMESTER 2
COMMON CORE (S2)
TU 201: ENGLISH AND SECOND LANGUAGE
Goals:
- To develop communication skills in a foreign language
- Specialised vocabulary
- Knowledge of the companies and health matters in the countries of the target language
- Cultural approach
Content:40hrs
- Work on all communication skills: written and oral expression and comprehension
- Knowledge of the professional and cultural international environment
- Job interview simulation, oral presentation using PowerPoint, meetings simulation, writing letters and emails, debates, documents reading etc.
- Methodological aspects (according to the groups’ needs)
- Practice on digital platform aiming at revising and strengthening language skills
Teachers:
- I. MAITRE-DEVALLON
- Y. ZAMBRANO
- G. NICKSON
- P. WEINMANN
TU 202: 3 to 5 MONTH WORK PLACEMENT
Goals:
- To put into practice the skills gained during the training course
- To achieve a concrete professional project within one’s context of activities
Content:
- Preparation and instruction to find and complete a work placement (3hrs-Lecture)
- 3 to 5 months full-time in a public or private company
Teachers:
- B. BAÑULS
- B. BATAILLE
- S. BEGU
- A. EL GHZAOUI
- P. RAVEL
- M. VIVIEN
- A. ESCANDE
- E. GOMEZ
- B. NOTTELET
- X. GARRIC
SPECIFIC TU (S2): MEDICAL DEVICES : FROM DESIGN TO MARKET
TU 215 :SYNTHESIS AND CHARACTERIZATION OF BIOMATERIALS
Objectives:
- Scientific and technical knowledge on the synthesis and characterization of biomaterials
Contenu:40h(Lecture) and 10h ( Practical work)
- Synthesis and characterization of biomaterials
- Mechanical and thermal properties
- Phisico-chemical properties
- Gel characterization
- Practical work and mini-projects
Speakers:
- J. COUDANE
- B. NOTTELET
- H. VAN DEN BERGHE
- X. GARRIC
- A. EL GHZAOUI
TU 216 : HEALTH APPLICATIONS OF BIOMATERIALS
Objectives:
- Knowledge of the criteria for the selection of biomaterials for health applications
- Regulatory and industrial constraints linked to applications of biomaterials in Health
- Knowledge of the different families of applications of biomaterials health products
- Ability to develop the specifications for an application
Content:35h (Lecture) and 15h (Practical work)
- Specifications and selection of the criteria with respect to the application
- Overview on degradation
- Surface modifications of medical devices
- Sterilization of medical devices
- Applications of biomaterials in medicine (tissue engineering, implantable medical devices …)
- Applications of biomaterials in Pharmacy
- Practical work and mini-projects
Speakers:
- B. NOTTELET
- X. GARRIC
TU 217 : LEARNING PROJECT: DESIGN AN MEDICAL DEVICE FROM BIOMATERIALS
Objectives:
- Develop autonomy and spirit of initiative of students to design a medical device
- Know the different stages of research and development required for the design of an innovative medical device
- Be able to identify a medical need and to analyze a market
- Be able to develop specifications adapted to the final application
- Suggest a design strategy
Content:70h (Tutorial)
Work plan :
- Literature review, scientific monitoring and competitive environment
- Medical and socio-economic context and issues
- Background and scientific issues
- Expected impact on medical, scientific and economic domains
- Regulatory aspects
Feasibility:
- Proposal of several design strategies based on a detailed analysis of the market and the scientific literature
- Budget needed :Raw materials(quantity, supplier, price, quality) /Equipment(quantity, supplier, price, quality) /Human resources
- Contact persons (Need to request information from third parties)
Practical work:
- Proposed design strategy and implementation thereof
Speakers:
- X. GARRIC
- B. NOTTELET
MASTER 2
SEMESTER 3
COMMON CORE (S3)
TU 301: HEALTH PRODUCTS PROJECT MANAGEMENT, MANAGEMENT CONTROL AND PERFORMANCE MANAGEMENT
Goals:
Project management:
- To understand the concepts, processes and project management methods within the frame described by the Project Management Institute
- Operational planning: from analysing a project to the preparation of a feasible work schedule
- To know the basic framework of innovation protection, manufacturing, the marketing and surveillance of medicines
Management control:
- To assess and understand through an account of company’s results, the items of profitality and performance
MS Project:
- To be able to use the MS Project software
Content:
- Project management (12hrs- Lecture + 12hrs-Tutorial)
- Management control and performance management (6hrs-Lecture + 6hrs-Tutorial)
- MS Project software (12hrs-Tutorial)
Teachers:
Montpellier University
- P. CREMADES
- R. BARTHES
External professionals
- Service provider
TU 302: QUALITY MANAGEMENT, MARKETING
Goals:
Team management:
- To clarify the missions, roles and function of a team manager (project)
- To understand the influence of one’s leadership of the team’s performance
- To identify one’s management style, one’s negotiation style
- To be able to lead a meeting
Quality management :
- To master quality management
Marketing:
- To understand the market’s mechanisms
- To be able to identify the relevant stakeholders and to analyse the influence factors
- To know the various strategic manoeuvres (by comparison with the demand and faced with the competition)
- To be able to define the appropriate operational modes
Content:
- Team management (12,5hrs-Tutorial)
- Quality management (14hrs-Lecture + 12hrs-Tutorial)
- Marketing (12hrs-Lecture)
Teachers:
- R. BARTHES
- N. DE LELIGNE
- L. KABBARA BARDINA
SPECIFIC TU: MEDICAL DEVICES : FROM DESIGN TO MARKET (S3)
TU 324 : OVERVIEWS AND GENERAL KNOWLEDGE ON MEDICAL DEVICES
Objectives:
- An introduction to the medical device sector
- Know the anatomical, histological, regulatory and health economic specificities of different medical disciplines that use medical devices
- Identify the major classes of medical devices by medical
- Understand the ways toward innovation in different medical specialities
Content:40h (Lecture) 10h (tutorial)
- Medical devices (Definition and introduction to the medical device sector)
- Biomaterials (Classification, Degradation, Stérilization)
- Classification of medical devices by medical disciplines
Speakers:
- X. GARRIC
- B. NOTTELET
- C. HIRTZ
- Hospital practitioners form Montpellier University Hospital Center
TU 325 : REGULATORY ASPECTS, STANDARDS AND MARKET ACCESS
Objectives:
- Acquire all general regulatory information for the development of medical devices
- Provide general and regulatory knowledge of the stages prior to marketing in the medico-economic evaluation plan and market access
Content:40h (Lecture) 10h (Tutorial)
- Regulations and Standards
- Market access
- CNEDiMTS (National Commission for assessment of medical devices and health technologies)
- Call for tenders in hospital
- Mini-project
Intervenants:
University of Montpellier
- V. RAGE-ANDRIEU
Montpellier University Hospital Center
- C. FAURE-CHAZELLES
- J. PERREY
External professionals
- Institutional speakers (ANSM, LNE, SNITEM, CNEDIMTS, Public and private hospitals…)
TU 326 : EVALUATION OF MEDICAL DEVICES
Objectives:
- Knowledge of the various steps and associated constraints of preclinical evaluation
- Theoretical and practical knowledge of the progress of a clinical trial dedicated to a medical device
- Knowledge of regulatory and administrative aspects of clinical trials
Content: 40h (Lecture) 10h (Tutorial)
- Biocompatibility, pre-clinical evaluation of medical device and compliance with the essential requirements
- Clinical trials of medical device
- Visit of site of preclinical and clinical evaluation service
- Mini-projects
Speakers:
University of Montpellier
- X. GARRIC
Montpellier University Hospital Center
- G. MERCIER
- A. CASTET
- Speakers from Medical Device Companies
TU 327 : MEDICAL DEVICES IN COMPANY – 1
Objectives:
- Present the different activities in company
- Explain the life cycle of the medical device in company
- Detail the various stages of the life cycle in company
- Understand how companies adapt regulatory aspects to design, produce and market medical devices
Content: 40h (Lecture) 10h (Tutorial)
- The different stages of the life cycle of medical devices
- The regulation of MDs (Objectives of demand and market analysis)
- Answers to the essential requirements
- Quality assurance
- Risk Analysis
- Manufacturing and Production
- Marketing
Speakers:
External professionals
- C. POMPEE (Aspide Medical)
- D. STEFANELLI (Gettinge group)
- A. GRANGE (Medtronic)
- F. DUPASQUIER (Bayer)
- M. DUMONT (Laboratoires Brothier)
SEMESTER 4
COMMON CORE –WORK EXPERIENCE PLACEMENT (S4)
TU 401: 4 TO 6 MONTH WORK PLACEMENT
Goals:
- To carry out a concrete professional project within its context of activities
Content:
- 2hrs Lecture: preparation and instructions to find and do a work placement
- 4 to 6 months full-time in a public or private company
Teachers:
- B. BAÑULS
- B. BATAILLE
- S. BEGU
- A. EL GHZAOUI
- P. RAVEL
- M. VIVIEN
- E. GOMEZ
- X. GARRIC
TU SPECIFIC: MEDICAL DEVICES – FROM DESIGN TO MARKET (S4)
TU 415 :MEDICAL DEVICES IN COMPANY – 2
Objectives:
- Know how to read or search a patent
- Understand all intellectual property rights and applied strategies
- Use project management during the life cycle of the medical device
- Know the tools to manage and control the production of medical devices
Content: 40h (Lecture) 10h (Practical work)
- Patent and intellectual Property
- Project management applied to the Medical Device
- Production management / Production control
- Practical work and mini-projects
- Industrial interventions : What is the job? What is the role in the company?
Speakers:
External speakers
- SNITEM speakers
- Others industrials
TU 416 : LEARNING PROJECT : THE LIFE CYCLE OF DM
Objectives:
- Develop autonomy andspirit of initiative of students for marketing a medical device
- Be able to identify a medical need and analyze a market
- Be able to develop specifications adapted to the application
- Know the different stages of the MD life cycle necessary to commercialize an innovative medical device in compliance with the quality approach
- Develop a marketing plan
Content:70h (Tutorial)
Work plan:
- Literature review, scientific monitoring and competitive environment
- Medical and socio-economic context and issues
- Background and scientific issues
- Expected impact on medical, scientific, and economic
- Regulatory aspects
Feasibility:
- Proposal of several design strategies based on a detailed analysis of the market and the scientific literature
- Budget needed
Practicality:
- Proposal of a design strategy
- Proposal of a production strategy
- Proposal of a marketing plan
Speakers:
University of Montpellier
- X. GARRIC
- B. NOTTELET
- C. HIRTZ